Mastering Polyatomic Ions: A Guide To Naming And Understanding

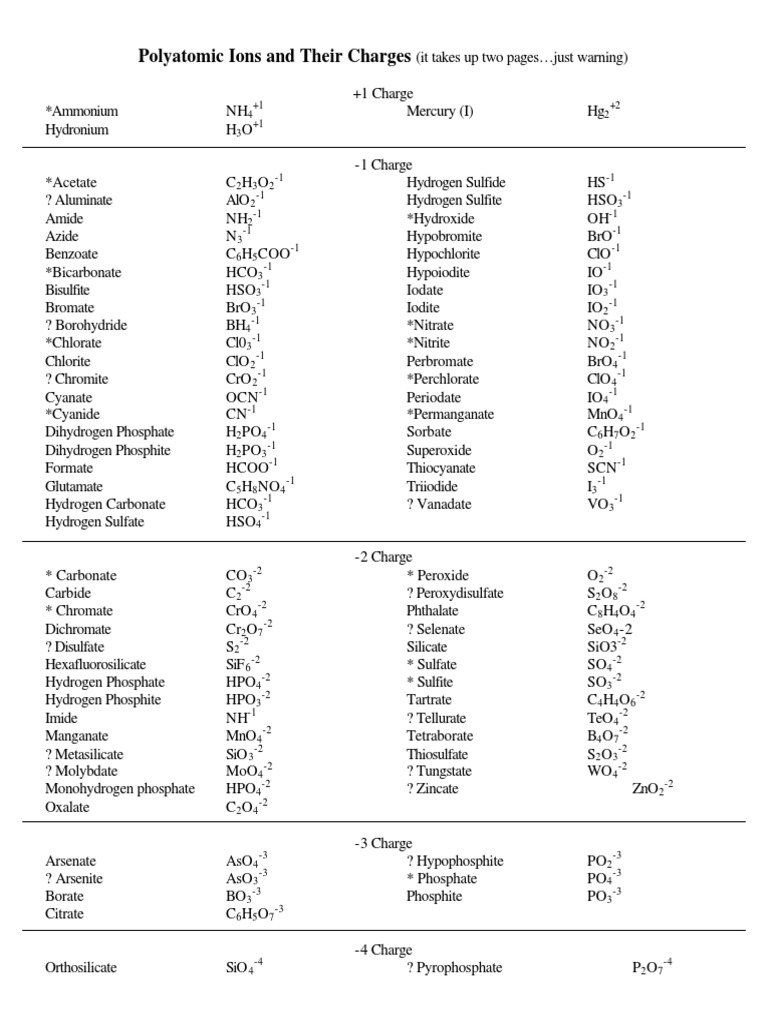
Polyatomic ions are an essential concept in chemistry, particularly in inorganic chemistry, where they play a crucial role in the formation of compounds and the understanding of chemical reactions. These ions, composed of multiple atoms, often exhibit unique behaviors and properties that are distinct from their individual constituent elements. In this comprehensive guide, we will delve into the world of polyatomic ions, exploring their naming conventions, structural characteristics, and the key insights they provide into the realm of chemical science.
The Intricate World of Polyatomic Ions

Polyatomic ions, as the name suggests, are ions that consist of two or more atoms bonded together. These ions carry a net electrical charge, which can be either positive (cations) or negative (anions). The unique aspect of polyatomic ions lies in their complex bonding patterns, where the atoms within the ion are held together by covalent or ionic bonds, or a combination of both.
One of the fundamental aspects of polyatomic ions is their ability to form compounds with other ions or elements. For instance, the polyatomic ion SO42- (sulfate ion) can combine with various cations to form a range of sulfate salts, such as MgSO4 (magnesium sulfate) or Na2SO4 (sodium sulfate). Understanding the behavior and reactivity of these ions is crucial for predicting and explaining the properties of the compounds they form.
Naming Polyatomic Ions: A Systematic Approach

Naming polyatomic ions follows a systematic and logical process, which is essential for clear communication and understanding in the field of chemistry. The naming conventions for polyatomic ions are based on the following guidelines:
1. Root Names and Suffixes
The name of a polyatomic ion typically begins with a root name that describes the central atom. This root name is derived from the name of the element, often with a prefix indicating the number of atoms. For example, the root name for the ion NO3- (nitrate ion) is nitr, derived from the element nitrogen. The suffix -ate is then added to indicate that the ion carries a negative charge.
Here's a table illustrating some common root names and their corresponding elements:
| Root Name | Element |
|---|---|
| sulf | sulfur |
| phosph | phosphorus |
| chlor | chlorine |
| ox | oxygen |

2. Charge and Oxidation State
The charge or oxidation state of a polyatomic ion is indicated by a numerical prefix or a suffix. For cations, the charge is often indicated by a Roman numeral in parentheses, following the root name. For anions, the charge is typically indicated by a suffix. For example, the ion Fe3+ (iron(III) ion) has a charge of +3, while the ion PO43- (phosphate ion) has a charge of -3.
3. Polyatomic Ion Groups
Polyatomic ions are often categorized into groups based on their structural and chemical properties. These groups include oxygen-containing anions, halogen-containing anions, and polyatomic cations. Understanding these groups can help in recognizing and naming common polyatomic ions.
Understanding Polyatomic Ions: Key Insights
Polyatomic ions offer valuable insights into the behavior of chemical compounds and reactions. Here are some key aspects to consider:
1. Chemical Properties
The chemical properties of polyatomic ions are determined by the nature of the bonds within the ion and the overall charge. For example, the OH- (hydroxide ion) is a strong base due to the presence of a highly reactive oxygen atom. Understanding these properties is crucial for predicting the behavior of compounds containing these ions.
2. Role in Chemical Reactions
Polyatomic ions are often involved in a wide range of chemical reactions. For instance, in acid-base reactions, polyatomic ions such as NO3- (nitrate ion) can act as spectator ions, remaining unchanged throughout the reaction. However, in redox reactions, polyatomic ions can undergo significant changes, leading to the formation of new compounds.
3. Structural Diversity
Polyatomic ions exhibit a remarkable structural diversity, with some ions forming complex three-dimensional structures. For example, the CO32- (carbonate ion) has a trigonal planar structure, while the SO42- (sulfate ion) has a tetrahedral structure. Understanding these structures is essential for predicting the shape and properties of the compounds they form.
Real-World Applications of Polyatomic Ions
Polyatomic ions have numerous real-world applications, particularly in the fields of environmental science, medicine, and industry. Here are a few examples:
1. Environmental Science
Polyatomic ions, such as NO3- (nitrate ion) and PO43- (phosphate ion), are crucial in understanding and addressing environmental issues. Excessive levels of these ions in water bodies can lead to eutrophication, a process that depletes oxygen levels and harms aquatic life. Monitoring and controlling the levels of these ions is essential for maintaining the health of ecosystems.
2. Medicine and Pharmacology
Polyatomic ions play a vital role in medicine and pharmacology. For instance, the ClO- (hypochlorite ion) is a key component of household bleach, used for disinfection and sanitization. Additionally, polyatomic ions are often used as active ingredients in medications, such as the CO32- (carbonate ion) in antacids, which helps neutralize excess stomach acid.
3. Industrial Processes
Polyatomic ions are essential in various industrial processes, including water treatment, where ions like SO42- (sulfate ion) and Cl- (chloride ion) are removed to produce potable water. Additionally, polyatomic ions are used in the production of fertilizers, where ions like NO3- (nitrate ion) and PO43- (phosphate ion) are key components, ensuring the growth and health of crops.
Conclusion: A Comprehensive Understanding
Polyatomic ions are a fascinating and complex aspect of chemistry, offering a wealth of insights into the behavior of chemical compounds and reactions. By understanding the naming conventions, structural characteristics, and key insights provided by polyatomic ions, we can gain a deeper appreciation for the intricate world of chemical science. Whether it’s predicting the properties of compounds, understanding chemical reactions, or addressing real-world environmental and industrial challenges, polyatomic ions play a pivotal role in our understanding of the chemical world.
What is the difference between a polyatomic ion and a simple ion?
+A polyatomic ion is composed of two or more atoms bonded together, carrying a net electrical charge. In contrast, a simple ion is a single atom that has gained or lost electrons, resulting in a net charge. Polyatomic ions are more complex in structure and often have unique chemical properties due to their multiple atoms.
How do polyatomic ions form compounds?
+Polyatomic ions form compounds by combining with other ions or elements. For example, the sulfate ion SO42- can combine with the cation Mg2+ to form the compound MgSO4 (magnesium sulfate). The negative charge of the polyatomic ion balances the positive charge of the cation, resulting in a neutral compound.
What are some common polyatomic ions and their charges?
+Some common polyatomic ions include NO3- (nitrate ion, charge -1), PO43- (phosphate ion, charge -3), SO42- (sulfate ion, charge -2), and OH- (hydroxide ion, charge -1). These ions are frequently encountered in various chemical reactions and compounds.