Polyatomic Ions Chart
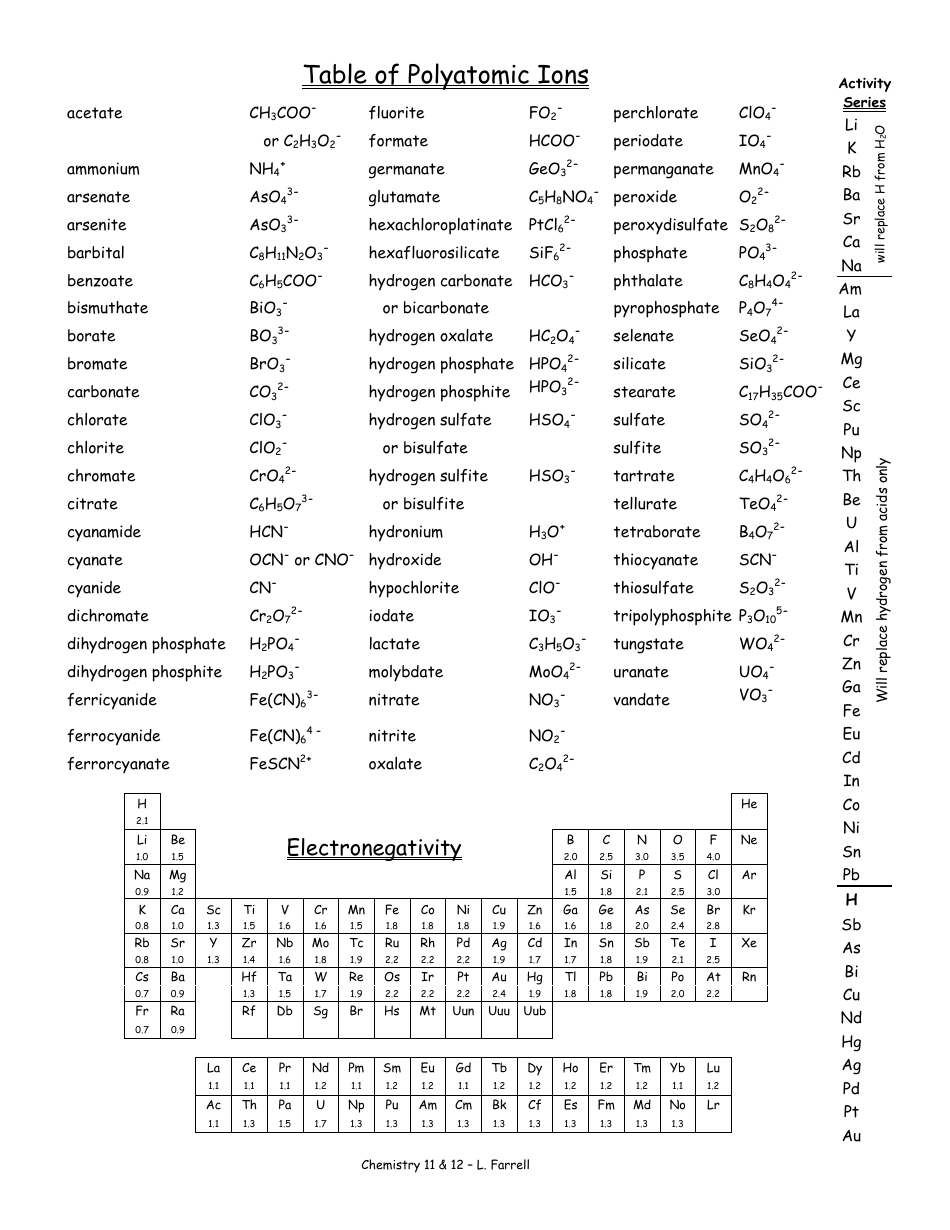
In the realm of chemistry, understanding the intricate world of polyatomic ions is essential for comprehending the fundamental building blocks of matter. Polyatomic ions, also known as molecular ions, are charged molecules consisting of two or more atoms. These ions play a crucial role in various chemical reactions and are fundamental to the structure and behavior of many compounds. This article aims to provide a comprehensive guide to polyatomic ions, exploring their properties, naming conventions, and their significance in the chemical world.
The Nature of Polyatomic Ions

Polyatomic ions are a diverse group of chemical species, each with its unique composition and charge. These ions are formed when a group of atoms bond together and acquire an overall electrical charge due to the gain or loss of electrons. The resulting ion can be either positively charged (cation) or negatively charged (anion), depending on the specific elements involved and the nature of the chemical bonding.
One of the key characteristics of polyatomic ions is their ability to form stable structures, often through covalent or ionic bonding. Covalent bonding involves the sharing of electrons between atoms, while ionic bonding occurs when one atom donates an electron to another, resulting in a positive and negative ion, respectively. This unique bonding behavior gives polyatomic ions their distinct properties and reactivity.
Examples of Polyatomic Ions
To illustrate the diversity of polyatomic ions, let’s explore a few common examples:
- Ammonium Ion (NH4+): This cation is formed when an ammonia molecule (NH3) accepts a proton (H+). It is a key player in many chemical reactions and is often found in acids and fertilizers.
- Nitrate Ion (NO3–): A common anion, the nitrate ion is composed of one nitrogen atom and three oxygen atoms. It is highly soluble in water and is a vital component in various chemical processes, including the nitrogen cycle.
- Sulfate Ion (SO42–): With a central sulfur atom surrounded by four oxygen atoms, the sulfate ion is another important anion. It is found in various minerals and plays a crucial role in biological processes.
- Carbonate Ion (CO32–): This anion, consisting of one carbon atom and three oxygen atoms, is often associated with carbon-containing compounds and is essential for the formation of limestone and other carbonate minerals.
These examples showcase the wide range of polyatomic ions and their importance in various chemical and geological contexts.
Naming and Formula Conventions

Naming polyatomic ions can be a complex task, as it involves a combination of systematic and traditional naming conventions. The systematic approach, favored by the International Union of Pure and Applied Chemistry (IUPAC), provides a consistent and logical system for naming ions based on their composition and charge.
Systematic Naming
The IUPAC naming system for polyatomic ions involves the following steps:
- Identify the Central Atom: The central atom is typically the atom with the highest electronegativity or the atom that forms the most bonds. In many cases, this is oxygen, nitrogen, or sulfur.
- Determine the Prefix: The prefix indicates the number of atoms of each element present in the ion. Common prefixes include mono-, di-, tri-, tetra-, and so on.
- Add the Root Name: The root name is derived from the central atom and indicates its presence. For example, "carbon" for carbon, "nitro" for nitrogen, and "sulf" for sulfur.
- Indicate the Charge: The charge of the ion is indicated by a suffix. For cations, the suffix is "-ium," while for anions, it is "-ide." The charge itself is not explicitly stated but can be inferred from the context.
For example, the sulfate ion (SO42–) would be named "sulfate" using the systematic approach.
Traditional Naming
Traditional naming conventions for polyatomic ions are often based on historical usage and may vary depending on the specific ion. These names are widely recognized and used in everyday chemistry.
Some common traditional names for polyatomic ions include:
- Ammonium (NH4+): Derived from ammonia, this name is used for the cation formed by protonation of ammonia.
- Nitrate (NO3–): This anion is named after the element nitrogen, with the suffix "-ate" indicating its acidic nature.
- Sulfate (SO42–): The name "sulfate" is derived from the element sulfur and is commonly used in chemical nomenclature.
- Carbonate (CO32–): The carbonate ion gets its name from the element carbon, with the suffix "-ate" indicating its acidic characteristics.
It's important to note that while traditional naming is widely accepted, the systematic IUPAC approach is increasingly favored for its consistency and clarity.
Chemical Properties and Reactivity
Polyatomic ions exhibit a wide range of chemical properties and reactivities, making them essential components in various chemical reactions. Their behavior is influenced by factors such as their charge, size, and the nature of the chemical bonds within the ion.
Acidic and Basic Properties
Many polyatomic ions, particularly anions, can act as either acids or bases in aqueous solutions. This behavior is governed by the ion’s ability to donate or accept protons (H+ ions). For example, the sulfate ion (SO42–) can act as a base by accepting a proton to form sulfuric acid (H2SO4).
On the other hand, cations like the ammonium ion (NH4+) can act as acids by donating a proton to form ammonia (NH3).
Solubility and Precipitation
The solubility of polyatomic ions in various solvents is a critical factor in chemical reactions and separations. Some polyatomic ions, such as the nitrate ion (NO3–), are highly soluble in water, while others, like the carbonate ion (CO32–), have limited solubility. This property is often exploited in precipitation reactions, where insoluble compounds are formed and can be separated from the solution.
Redox Reactions
Polyatomic ions can also participate in redox (reduction-oxidation) reactions, where they gain or lose electrons. For instance, the sulfate ion (SO42–) can be reduced to the sulfite ion (SO32–) by gaining two electrons. Redox reactions are crucial in various chemical processes, including electrochemistry and biological systems.
Polyatomic Ions in Biological Systems
Polyatomic ions play a vital role in biological processes, where they are involved in essential functions such as nutrient transport, enzyme activity, and maintaining the body’s pH balance.
Nutrient Transport
Certain polyatomic ions, such as the phosphate ion (PO43–), are essential for the transport of nutrients across cell membranes. Phosphate ions, for example, are crucial for the synthesis and storage of energy in the form of ATP (adenosine triphosphate) in living organisms.
Enzyme Activity
Enzymes, the biological catalysts that facilitate chemical reactions in living organisms, often require the presence of specific polyatomic ions for their activity. For instance, the zinc ion (Zn2+) is a crucial cofactor for many enzymes, including carbonic anhydrase, which plays a key role in maintaining the body’s pH balance.
pH Regulation
Polyatomic ions, particularly those involved in acid-base chemistry, are essential for regulating the pH of biological fluids. For example, the bicarbonate ion (HCO3–) is a key player in the body’s pH buffering system, helping to maintain a stable pH in the blood and other bodily fluids.
Environmental and Industrial Significance

Polyatomic ions are not only important in biological systems but also have significant environmental and industrial implications.
Environmental Chemistry
The study of polyatomic ions in environmental chemistry focuses on their role in natural processes and their impact on the environment. For example, the nitrate ion (NO3–) is a critical component of the nitrogen cycle, which is essential for the growth of plants and the maintenance of ecological balance.
However, excessive nitrate levels in water bodies can lead to eutrophication, a process where excessive nutrient levels stimulate the growth of algae and other aquatic plants, leading to oxygen depletion and the death of aquatic life.
Industrial Applications
Polyatomic ions are utilized in various industrial processes, from the production of fertilizers to the synthesis of chemicals. For instance, the ammonium ion (NH4+) is a key component in the manufacture of nitrogen-based fertilizers, while the sulfate ion (SO42–) is essential for the production of sulfuric acid, a vital industrial chemical.
Challenges and Future Research
While our understanding of polyatomic ions has advanced significantly, there are still many challenges and areas for future research. Some of the key areas of focus include:
- Ion Structure and Bonding: Understanding the intricate bonding patterns and structures of polyatomic ions can provide valuable insights into their reactivity and behavior.
- Environmental Impact: Further research is needed to assess the environmental impact of various polyatomic ions, particularly in the context of pollution and climate change.
- Biological Functions: Exploring the specific roles of polyatomic ions in biological systems can lead to advancements in medicine and biotechnology.
- Industrial Innovations: Developing new processes and technologies that utilize polyatomic ions more efficiently and sustainably can drive industrial innovation.
As our knowledge of polyatomic ions continues to evolve, we can expect further advancements in chemistry, biology, and various industries, leading to a deeper understanding of the building blocks of matter and their applications.
Conclusion
Polyatomic ions are an integral part of the chemical world, playing diverse and critical roles in various fields. From their naming conventions and chemical properties to their biological and environmental significance, these ions are a testament to the complexity and beauty of the chemical universe. As we continue to explore and understand these ions, we unlock new possibilities for innovation and discovery, shaping the future of science and technology.
How do polyatomic ions differ from monatomic ions?
+Polyatomic ions consist of two or more atoms bonded together and carry an overall electrical charge, while monatomic ions are single atoms that have gained or lost electrons, resulting in a charge.
What is the significance of polyatomic ions in environmental chemistry?
+Polyatomic ions, such as nitrate and sulfate ions, play a crucial role in natural processes like the nitrogen cycle and can have significant environmental impacts, including eutrophication.
How are polyatomic ions named using the systematic IUPAC approach?
+The IUPAC naming system involves identifying the central atom, using prefixes to indicate atom numbers, adding root names, and using “-ium” for cations and “-ide” for anions.



